Running head: CENTRAL PLACE FORAGING
THEORY AND PREY DISTRIBUTION
CENTRAL-PLACE FORAGING THEORY
AND
AN ABRUPT PREY DISTRIBUTION LIMIT
John Landahl
Keywords: mussels, Mytilus edulis.
ABSTRACT
Although central-place foraging theory was
originally developed for the case in which an individual predator
forages out in all directions from a central location of some
sort, its conclusions can be generalized to accommodate predator
populations foraging out of refuges, not necessarily in all
directions, and to infer the effects of their foraging on prey
distributions within habitats. Other investigations have dealt
with aspects of central-place foraging theory as it relates to
predator behavior, but the present study indicates that its
implications for prey distribution are also of interest.
INTRODUCTION
Well-defined (though often irregular) limits of
the distributions of plants and of sessile animals within
habitats (as opposed to larger geographic areas) are periodically
observed in nature, sometimes in the absence of any obvious
corresponding environmental discontinuity (e.g.,the lower margin
of the mussel bed at Quartermaster Harbor described in Landahl
1985, Chapter III). In several cases experimental studies have
demonstrated that distinct distributional boundaries occur at the
border between partially overlapping predator and prey
distributions. A well-known example is the lower limit of M.
californianus in the rocky intertidal zone on the outer coast
of Washington; this limit is set by sea star predation (Paine,
1974, 1976). From a theoretical standpoint, spatial distributions
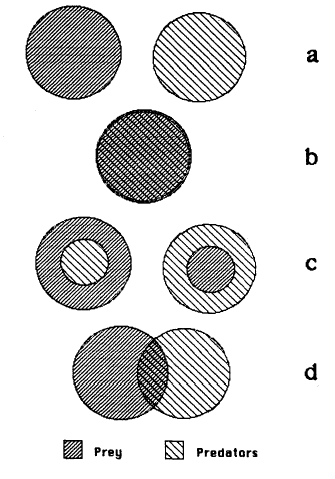
of predators and prey in a habitat can be disjoint or can overlap
in several different ways (Figure 1) . When
the predator and prey distributions are neither disjunct nor
completely overlapping, the potential exists for the foraging
activities of the predators to reduce prey density within a
subset of the prey distribution.

Figure 1. Classes of within-habitat
predator and prey distributions: disjoint (a), congruent (b),
concentric with prey outside (c left), concentric with
prey inside (c right), and overlapping (d).
In terrestrial systems, zones bare of vegetation
have long been noted around shrubs in the California chaparral
and coastal sage communities (e.g.,Whittaker, 1975; Ricklefs,
1980). These zones were originally interpreted as areas in which
volatile toxins (e.g.,terpenes) produced by these shrubs (which
include Salvia leucophylla, Adenostoma fasciculatum,
Artemisia californica, and Baccharis pilularis)
inhibited the growth of germinating seedlings (e.g.,Muller,
Muller, and Haines, 1964; Muller and del Moral, 1966). However,
Bartholomew (1970) used herbivore exclosures to show that these
bare zones are maintained by the feeding activity of rodents,
rabbits, and birds which take shelter in the shrubs but emerge
(particularly at night) to browse or eat seeds in the surrounding
grasslands.
Similar bare zones 1-2 m in width around clumps of
the Argentinean thornbushes Schinus fasciculatus, Condalia
microphylla, and Lycium gilliesianum are maintained by
the foraging and trampling activities of the desert cavy (Microcavia
australis), a small, ground-dwelling hystricomorph rodent
(Rood, 1970).
Suchanek (1978, 1979) reported that distinct
"halos" bare of the seaweed Fucus surround
isolated mussel beds on rock hillocks in this habitat. Using
field experiments, he showed that these zones, which are usually
about 20 cm wide, were produced by the grazing activities of
limpets which, at low tide, take shelter from desiccation within
the mussel beds.
In the course of other studies (Landahl 1985,
Chapter III), it became apparent that the intertidal mussel
population at Quartermaster Harbor had an abrupt lower
distributional boundary which did not coincide with any
pronounced discontinuity in substrate (Landahl 1985). Experiments
showed that this boundary was the result of predation by the kelp
crab Pugettia producta (Landahl 1985, Chapter III).
However, the reason for the abruptness of a boundary such as this
was less clear. Woodin (1976) has suggested that intense
adult-larval interactions can produce sharp boundaries between
dense infaunal assemblages dominated by organisms of different
functional types (e.g.,suspension-feeders, deposit-feeders). It
seems likely that this mechanism could also produce sharp
boundaries between dense beds of epifauna as well.
Hence one might hypothesize that the sharp lower
margin of the mussel bed reflects the juncture between a dense
epifaunal assemblage and a dense infaunal one; this will be
referred to as the adult-larval interaction hypothesis. However,
populations of infauna (or at least of larger infaunal bivalves)
were not dense below the lower margin of the mussel beds (Landahl
1985, Chapter III), so the adult-larval interaction hypothesis
does not appear satisfactory to explain the sharpness of the
mussel distribution boundary.
An alternative hypothesis (here termed the
"physiological tolerance" hypothesis) is that the
predator's upper foraging limit is set by its physiological
tolerances, and this hypothesis seems reasonable in the case of
slow-moving predators susceptible to rapid desiccation (e.g.,sea
stars). It is not surprising that the foraging activities of
slow-moving predators poorly equipped to withstand desiccation
(e.g.,sea stars, sea urchins) would set a distinct lower boundary
for an intertidal mussel population as a result of their own
physiological tolerances, but, for a variety of reasons, it is
surprising that crab predation would set a similarly abrupt
margin.
Unlike Evasterias troschelii and other sea
stars known to prey on bivalves (Mauzey, Birkeland, and Dayton,
1968), P. producta moves rapidly. It is also highly
resistant (though not completely invulnerable) to short-term
desiccation by virtue of its chitinous exoskeleton. For this
reason the upper limit of its foraging activities is not likely
set by physiological tolerances. The width of the mussel bed at
my study area was approximately 120 m (Landahl 1985). It is
difficult to imagine that a sea star could traverse such a
distance and return to the lower intertidal zone within one tidal
cycle, but it is quite possible that a crab could migrate daily
in this way if it were advantageous to do so. Carcinus maenas,
a predatory crab reaching 7 cm in carapace width (Dare and
Edwards, 1981), is capable of travelling up to 300 m upshore
during high tides (Dare and Edwards, 1981). The portunid crab Scylla
serrata found in S. Africa and Australia can move as much as
800 m during one night (Hill, 1978). Adult C. magister in
Similk Bay, Wash., are estimated to move 92 m/day on the average
(Mayer, 1973). Mass movements of crabs upshore on rising tides
have been observed using underwater television (Dare and Edwards,
1981); corresponding movements downshore when the falling tide
threatens to expose these animal also occur (Dare and Edwards,
1981).
Scoter ducks (which overwintered at Quartermaster
Harbor; Chapter V) are known to prey on crabs (Cottam, 1939), and
10 to 20 Glaucous-winged Gulls and two Great Blue Herons were
also present at this site for much of the year (Chapter V). In
studies conducted in the Strait of Juan de Fuca and other inland
marine waters of the State of Washington, Hirsch (1980) found
that the diet of the Common Goldeneye (Bucephala clangula)
included crabs of the genus Cancer and that the diet of
the Oldsquaw (Clangula hyemalis) included P. producta.
She found crustaceans of several species and unidentified crab
claws in stomachs of White-winged and Surf Scoters, suggesting
that, like Common Goldeneyes and Oldsquaws, the scoters may prey
on these crabs as well.
Crabs may also be eaten by fish (e.g.,rockfish,
sculpins) (Hines, 1982). However, rockfish and sculpins are not
usually found in sandy or muddy areas, and only flatfish and
salmon were seen during daytime snorkeling at Quartermaster
Harbor.
Thus bird predation could have been expected to
force crabs to retreat into the lower intertidal or the subtidal
zones during daylight hours, and in fact I seldom observed crabs
in the mussel bed during daylight snorkeling. In this case, the
predators presumably only move far enough downward to escape
their own predators, which are inferred to be diving ducks and
Great Blue Herons. P. producta reaches densities of 1-2/m2 in a band comprising the rocky intertidal and shallow
seagrass zones along the shore on the coast of California, but is
almost absent in deeper water (Hines, 1982). At Quartermaster
Harbor, P. producta could sometimes be found completely or
partially burrowed into the sand in shallow water during daylight
low tides, a behavior which suggested avoidance of visual
predators. However, there was no obvious reason why crabs could
not have invaded the interior of the mussel bed at night if they
chose, and indeed, occasional instances of crab predation on
mussels were observed well upshore of the lower margin.
A second alternative hypothesis (here termed the
central-place foraging hypothesis) which would explain the
abruptness of prey distribution boundaries resulting from
predation is based on central-place foraging theory (Orians and
Pearson, 1979; Orians, 1980). A central place, such as a nest,
den, or colony site, is a location to which a predator returns
after each foraging trip to store food, or feed young (Andersson,
1981). Central-place foraging theory is concerned with the
energetic cost of travelling between this location and the
foraging area and the amount of energy gained during the foraging
period (Orians and Pearson, 1979; Orians, 1980).
Although central-place foraging theory was
originally developed for the case in which an individual predator
forages out in all directions from a central location of some
sort, and has been applied primarily to aspects of foraging
behavior such as patch choice and cheek-pouch loading in the
Eastern chipmunk, Tamias striatus (Kramer and Nowell,
1980), search area in the Whinchat, Saxicola rubetra
(Andersson, 1981), and hunting method choice and patch use in the
American Kestrel, Falco sparverius (Rudolph, 1982), its
conclusions can be generalized to accommodate predator
populations foraging out of refuges, not necessarily in all
directions, and to infer the effects of their foraging on prey
distributions within habitats.
The central-place foraging hypothesis relating to
prey distribution states that when a predator (or, presumably, a
predator population) has a refuge or other central place (e.g a
nest) within an otherwise uniform habitat which is occupied by a
prey species, the foraging activities of that population will
create a distinct and expanding zone largely or entirely free of
optimal-size prey within a uniform distance from the refuge
(Figure 2a). Woodin (1978) has discussed the role of refuges in
ecological systems in detail.
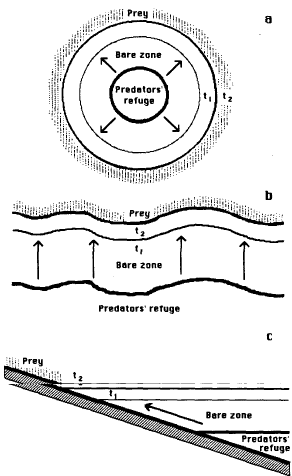
Figure 2. Maintenance and expansion of a
sharp-boundaried bare zone of uniform width by the foraging
activities of a predator population utilizing a refuge. Arrows
show the predators' outward movements during foraging; t1 is the width of the zone at time 1 and t2 is the width at time 2. When the refuge is a small
central place, the bare zone is circular (a). When the
refuge is the shallow subtidal zone along a shoreline, the shape
of the bare zone is as shown in top view (b) and in
cross-section (c).
The mechanism hypothesized to produce the
uniformity of this bare zone ("halo" or "browse
zone", in the terminology of Suchanek, 1978, 1979), with its
sharp boundary, is foraging by the predator in a manner which
minimizes its round-trip travel time and travel costs.
A case of this hypothesized phenomenon of
particular interest to marine ecologists occurs when a predator
on a sessile intertidal prey species takes periodic refuge from
desiccation, from predation, or from both in the lower intertidal
zone or in the shallow subtidal zone, resulting in a well-defined
prey distribution boundary (Figure 2b,c). It has not been shown,
however, that the upper limit of foraging by a predator taking
periodic refuge in the lower intertidal zone is a result of
minimizing either the time or cost of round-trip travel.
However, the central-place foraging hypothesis as
applied to the intertidal zone (stating that lower intertidal
predators forage upshore in such a way as to minimize round-trip
travel time, or perhaps to minimize total feeding time; Schoener,
1971), appeared more satisfactory than the adult-larval
interaction hypothesis or the physiological tolerance hypothesis
to explain the abrupt M. edulis distribution boundary near
the bottom of the middle intertidal zone at Quartermaster Harbor.
Discussion
The foraging behavior of P. producta has
been little studied. However, Elner and Hughes (1978) studied
foraging by C. maenas on isolated specimens of M.
edulis in England in some detail. P. producta is a
majid crab, while C. maenas is a cancrid. However, since
the body sizes of the two species are similar, it is likely that
the results obtained by Elner and Hughes (1978) can be
extrapolated to P. producta. They concluded that C.
maenas is a tactile predator, making use of neither
chemosensory nor visual cues in locating its prey. S. serrata,
on the other hand, is reported to detect infaunal and epifaunal
bivalves by contact chemoreception, the dactyls of the walking
legs being the location of the sensory receptors involved (Hill,
1979). Lack of reliance on visual cues is, of course, to be
expected in nocturnal predators.
Elner and Hughes (1978) also found that for a crab
of a particular size there is a mussel which is the optimal-size
prey. Feeding on mussels of this size minimizes handling time and
maximizes energy gain, smaller mussels having less energy content
and larger ones taking disproportionately longer to handle and
consume. It is likely that a P. producta of a given size
also has an optimal prey size even when feeding on mussels in
clumps rather than on isolated individuals.
However, because crabs have a wide repertoire of
attack modes (Elner and Hughes, 1978; Williams, 1978; Seed,
1982), there is no maximum-size mussel within the length range
1-6.5 cm which an individual C. maenas cannot consume
(Elner and Hughes, 1978). By resorting to edge-breaking (a slow
attack mode) rather than crushing (a fast attack mode), even very
large prey can be consumed. Since mussels at Quartermaster Harbor
did not exceed 70 cm in shell length (Landahl 1985) and P.
producta proved capable of consuming mussels at least 57 mm
long in the laboratory, it is likely that the mussels at this
locality had no size refuge from crab predation.
C. maenas adjusts rapidly to the prey size
distribution it encounters in its foraging activities (Elner and
Hughes, 1978). Thus crab predation would not necessarily be
size-selective over a long time-span, since less-preferred prey
would be consumed once the optimal-size mussels had been eaten. S.
serrata also consumes a wide size-range of prey (Hill, 1979).
Most mussels at Quartermaster Harbor occurred in
clumps, though a few isolated individuals were also present.
Observations of feeding by a P. producta in a lab tank
indicated that one of the first steps taken by this predator in
attacking a mussel in a clump is use its chelae to snip the
byssal threads attaching it to other mussels. This was sometimes
a time-consuming process and could have resulted in a significant
increase in handling time over that required for the isolated
individual mussels used as prey items by Elner and Hughes (1978).
Portunus puber also cuts the byssal threads of M.
edulis to separate individuals from clumps, and Scylla
serrata, a large Indo-West-Pacific portunid crab, likewise
begins its attack on hairy mussels (Trichomya hirsuta)
attached to rocks by breaking the byssal threads (Williams,
1978).
However, P. producta has smaller and less
robust chelae than C. maenas. Elner (1980) investigated
the effect of master chela size on the prey selection by C.
maenas. C. maenas is sexually dimorphic in master
chela size, with females possessing smaller master chelae than
males of the same carapace width (Elner, 1980). However, some
males also possess small master chelae, apparently as a result of
accidental loss of the master chela as juveniles (Elner, 1980).
Possession of a small master chela reduces the preferred size
prey for a C. maenas of a given body size, suggesting that
the optimal prey size for a P. producta may be smaller
than for a C. maenas of the same carapace width or tissue
dry weight. However, the conclusions of Elner and Hughes (1978)
should still be applicable to P. producta in a qualitative
way.
The effect of C. maenas predation on the
size structure of mussel populations has not been established
(Elner, 1980), although field experiments have shown that C.
maenas predation influences mussel distribution in Great
Britain (Kitching, Sloane, and Ebling, 1959; Ebling et al., 1964;
Elner, 1980). C. maenas predation on the dogwhelk (Nucella
lapillus) and on two littorinid snails (Littorina rudis
and L. nigrolineata) is not size-selective due to the
sparse distributions and unpredictable vulnerability of these
prey (Hughes and Elner, 1979; Elner and Raffaelli, 1980).
At Quartermaster Harbor, the largest mussels in
the bed were found at or near the lower margin (Landahl 1985), a
common pattern in intertidal molluscs (Vermeij, 1972). The lower
margin was also the optimal patch type for crab foraging because
the mussels population was densest there (Landahl 1985) and
because the mussels occurred in clumps which usually contained a
full range of sizes from juveniles to adults of maximum size,
presumably because some recruitment occurred at almost all times
of year (Landahl 1985). Thus, no matter what the optimal-size
mussel was for a given crab, that size would be encountered at
the lower margin of the mussel bed. Moving higher would
completely eliminate the possibility of encountering the largest
prey, and would not greatly increase the probability of
encountering prey of small or intermediate sizes. Small crabs
could have foraged equally profitably at higher levels. However,
since crabs retreated below the lower margin of the mussel bed
during daylight hours and during low tides, foraging at the lower
margin of the bed would have minimized travel time and travel
costs for crabs of all sizes. It was also probable that it was
advantageous for crabs to move the minimum possible distance
upshore to feed in order to minimize the risk of predation on
them by birds.
The hypothesis that P. producta foraged
shoreward from its refuge in such a way as to minimize its
round-trip travel time and costs or its total feeding time
appeared to provide a more satisfactory explanation of the
observations. It is difficult to distinguish between energy
maximizing and time minimizing behavior in studies of crab
foraging (Hughes and Seed, 1981). However, in future experiments
it might be possible to differentiate between these two
strategies by tethering crabs in the intertidal zone to determine
whether they are subject to predation, e.g.,by birds such as
scoters or great blue herons, and hence would benefit from
minimizing the time spent feeding in that zone. When predators
are themselves subject to predation by animals at higher trophic
levels, the element of risk must be considered and
time-minimizing behavior is expected to be favored over
energy-maximizing behavior.
P. producta's preference for mussels near
the lower margin of the bed at Quartermaster Harbor was
parsimoniously explained on the basis of central-place foraging
theory. Thus central-place foraging theory has utility in the
study of marine ecological systems. In this case, it offered an
explanation for two major static features of the mussel bed (the
existence of the lower bed margin and its distinctness) and one
dynamic aspect (the retreat of the boundary over time). It may be
applicable to other situations as well. For example, the sea
urchin Centrostephanus coronatus is another mobile marine
invertebrate which forages at night to avoid predation (by the
sheephead wrasse, Pimelometopon pulchrum, in this case)
(Nelson and Vance, 1979), and also minimizes its travel time by
staying near its refuge (holes and crevices in rock).
These results are of theoretical interest because
they suggest that even when habitats lack structural refuges for
prey (e.g.,holes, crevices, caves) and physical barriers which
might impede the movement of predators, optimal foraging
(e.g.,time-minimization or energy maximization) may result in the
focussing of predation on a restricted portion of the prey
habitat, producing phenomena such as the abrupt prey distribution
limit at Quartermaster Harbor. General predator-prey models in
the Lotka-Volterra lineage (e.g.,MacArthur, 1972; May, 1976) do
not predict such abrupt prey distribution boundaries in the
absence of physical barriers to prey or predator movement.
Other investigations (e.g.,Orians and Pearson,
1979; Orians, 1980; Kramer and Nowell, 1980 Andersson, 1981;
Rudolph, 1982) have dealt with aspects of central-place foraging
theory as it relates to predator behavior, but the present study
indicates that its implications for prey distribution are also of
interest.
Literature cited
Andersson, M. 1981. Central place foraging in
the whinchat, Saxicola rubetra. Ecology 62: 538-544.
Bartholomew, B. 1970. Bare zone between California
shrub and grassland communities: the role of animals. Science
170: 1210-1212.
Boulding, E. G. 1984. Crab-resistant features of
shells of burrowing bivalves: decreasing vulnerability by
increasing handling time. J. Exp. Mar. Biol. Ecol. 76. In press.
-----, and T. K. Hay. 1984. Crab response to prey
density can result in density-dependent mortality of clams. Can.
J. Fish. Aquat. Sci. 41. In press.
Cottam, C. 1939. Food habits of North American
diving ducks. U. S. Dept. Agri. Tech. Bull. 643. 139 p.
Dare, P. J., and D. B. Edwards. 1981. Underwater
television observations on the intertidal movements of shore
crabs, Carcinus maenas, across a mudflat. J. Mar. Biol.
Assn. U. K. 61:107-116.
Ebling, F. J., J. A. Kitching, L. Muntz, and C. M.
Taylor. 1964. The ecology of Lough Ine. XIII. Experimental
observations on the destruction of Mytilus edulis and Nucella
lapillus by crabs. J. Anim. Ecol. 33: 73-82.
Elner, R. W. 1980. The influence of temperature,
sex, and chela size in the foraging strategy of the shore crab, Carcinus
maenas (L.). Mar. Biol. Physiol. 7: 15-24.
-----, and R. N. Hughes. 1978. Energy maximization
in the diet of the shore crab Carcinus maenas. J. Anim.
Ecol. 47: 103-116.
-----, and D. G. Raffaelli. 1980. Interactions
between two marine snails, Littorina rudis Maton and Littorina
nigrolineata Gray, a predator, Carcinus maenas (L.),
and a parasite Microphallus similis Jägerskiold. J. Exp.
Mar. Biol. Ecol. 43: 151-160.
Hill, B. J. 1978. Activity, track and speed of
movement of the crab Scylla serrata in an estuary. Mar.
Biol. 47: 135-141.
-----. 1979. Aspects of the feeding strategy of
the predatory crab Scylla serrata. Mar. Biol. 55: 209-214.
Hines, A. H. 1982. Coexistence in a kelp forest:
size, population dynamics, and resource partitioning in a guild
of spider crabs (Brachyura, Majidae). Ecol. Monogr. 52: 179-198.
Hirsch, K. V. 1980. Winter ecology of sea ducks in
the inland marine waters of Washington. Seattle, WA: Univ. of
Wash. 92 p. Thesis.
Hughes, R. N., and R. W. Elner. 1979. Tactics of a
predator, Carcinus maenas, and morphological responses of
the prey, Nucella lapillus. J. Anim. Ecol. 48: 65-78.
-----, and R. Seed. 1981. Size selection of
mussels by the blue crab Callinectes sapidus. Mar. Ecol.
Prog. Ser. 6: 83-89.
Kitching, J. A., J. F. Sloane, and F. J. Ebling.
1959. The ecology of Lough Ine. VIII. Mussels and their
predators. J. Anim. Ecol. 28: 331-341.
Kramer, D. L., and W. Nowell. 1980. Central place
foraging in the eastern chipmunk, Tamais striatus. Anim.
Behav. 28: 772-778.
Landahl, J. T. 1985. Patterns of distribution of Mytilus
edulis, their causes, and their effects on co-occurring fauna
of a sand-gravel beach. Seattle, WA: Univ. of Wash. Dissertation.
337 p.
Landahl, J. T. 1988. Sediment-level fluctuation in
a mussel bed on a protected sand-gravel beach. Estuarine,
Coastal, and Shelf Science 26: 255-267. Keywords: sediment
movement, mussels, Mytilus, storms.
MacArthur, R. H. 1972. Geographical ecology:
patterns in the distribution of species. New York: Harper and
Row. 269 p.
Mauzey, K. P., C. Birkeland, and P. K. Dayton.
1968. Feeding behavior of asteroids and escape responses of their
prey in the Puget Sound region. Ecology 49: 603-619.
May, R. M. (ed.) 1976. Theoretical ecology:
principles and applications. Philadephia: W. B. Saunders Co. 317
p.
Mayer, D. L. 1973. The ecology and thermal
sensitivity of the Dungeness crab, Cancer magister, and
related species of its benthic community in Similk Bay,
Washington. Seattle, WA: Univ. of Wash. Dissertation. 188 p.
Muller, C. H., W. H. Muller, and B. L. Haines.
1964. Volatile growth inhibitors produced by aromatic shrubs.
Science 143: 471-473.
-----, and R. del Moral. 1966. Soil toxicity
induced by terpenes from Salvia leucophylla. Bull. Torrey
Bot. Club 93: 130-137.
Nelson, B. V., and R. R. Vance. 1979. Diel
foraging patterns of the sea urchin Centrostephanus coronatus
as a predator avoidance strategy. Mar. Biol. 51: 251-258.
Newcombe, C. L. 1935. A study of the community
relationships of the sea mussel, Mytilus edulis L. Ecology
16: 235-243.
Orians, G. H. 1980. Some adaptations of
marsh-nesting blackbirds. Monographs in population biology No.
14. Princeton, N. J.: Princeton Univ. Press. 295 p.
-----, and N. E. Pearson. 1979. On the theory of
central place foraging. Pages 155-177 in D. H. Horn, R. Mitchell,
and G. R. Stairs (eds.). Analysis of ecological systems.
Columbus, Ohio: Ohio State Univ. Press.
Paine, R. T. 1974. Intertidal community structure:
experimental studies on the relationship between a dominant
competitor and its principal predator. Oecologia 15: 93-120.
-----. 1976. Size-limited predation: an
observational and experimental approach with the Mytilus-Pisaster
interaction. Ecology 57: 858-873.
Ricklefs, R. E. 1979. Ecology (second ed.). New
York: Chiron. 966 p.
Rood, J. P. 1970. Ecology and social behavior of
the desert cavy (Microcavia australis). Am. Midland Nat.
83: 415-454.
Rudolph, S. G. 1982. Foraging strategies of
American Kestrels during breeding. Ecology 63: 1268-1276.
Schoener, T. W. 1971. Theory of feeding
strategies. Ann. Rev. Ecol. And Syst. 2: 369-404.
Seed, R. 1982. Predation of the ribbed mussel Geukensia
demissa by the blue crab Callinectes sapidus. Neth. J.
Sea Res. 16: 163-172.
Suchanek, T. H. 1978. The ecology of Mytilus
edulis L. in exposed rocky intertidal communities. J. Exp.
Mar. Biol. Ecol. 31: 105-120.
-----. 1979. The Mytilus californianus
community: studies on the composition, structure, organization,
and dynamics of a mussel bed. Seattle, WA: Univ. of Wash.
Dissertation.
Vermeij, G. J. 1972. Intraspecific shore-level
size gradients in intertidal molluscs. Ecology 53: 693-700.
Whittaker, R. H. 1975. Communities and ecosytems
(2nd ed.). New York: Macmillan. 385 p.
Williams, M. J. 1978. Opening of bivalve shells by
the mud crab Scylla serrata Forskal. Aust. J. Mar.
Freshwater Res. 29: 699-702.
Woodin, S. A. 1976. Adult-larval interactions in
dense infaunal assemblages: patterns of abundance. J. Mar. Res.
34: 25-41.
-----. 1978. Refuges, disturbance, and community
structure: a marine soft-bottom example. Ecology 59: 274-284.
Original draft completed 30-Apr-84
Last substantive revision 10/7/90
HTML version last updated 11/4/97